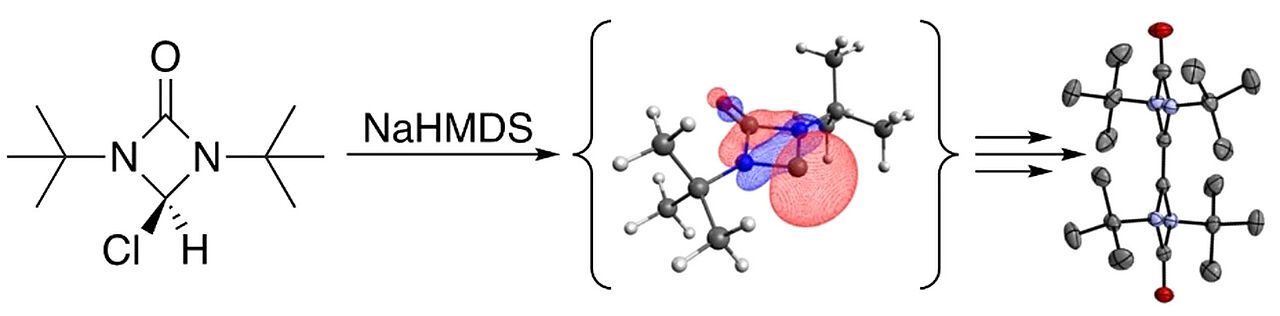
Attempts towards the synthesis of two novel four-membered 1,3-diazetidine based N-heterocyclic carbenes (NHCs) containing an organic backbone with a carbonyl functionality were undertaken. These carbenes cannot be isolated but the respective carbene dimers are obtained in quantitative yield which undergo a degradation and rearrangement sequence upon thermal exposure. Some of the species involved in these thermal reactions could be isolated and characterized, others were observed by mass spectrometric experiments. Ab initio and density functional theory (DFT) calculations provide a mechanistic rationale for the experimental observations. Since dimerization is strongly favored, classic carbene trapping reactions remain a goal to achieve.
Read more in:
Investigations on Novel 1,3-Diazetidine Based Four-Membered N-Heterocyclic Carbenes
Leonard Karl, Prof. Dr. Jan Meisner, Prof. Dr. Christian Ganter
Eur. J. Inorg. Chem, 26, 2023, e202300022
https://doi.org/10.1002/ejic.202300022